Back to Annual Meeting Program
Early duplex predicts late restenosis after renal artery angioplasty and stenting
Jason W Christie, Thomas D Conlee, Timothy E Craven, Justin B Hurie, Kimberley J Hansen
Wake Forest University Baptist Medical Center, Winston-Salem, NC
INTRODUCTION:
To examine the relationship between early duplex sonography and restenosis after primary renal artery percutaneous angioplasty and stenting (RA-PTAS).
METHODS:
Consecutive patients undergoing RA-PTAS for hemodynamically significant atherosclerotic renal artery stenosis (RAS) with hypertension and/or ischemic nephropathy between September 2003 and July 2010 were identified from a prospective registry. Patients had renal artery duplex ultrasound (RDS) pre RA-PTAS, within one week of RA-PTAS and follow-up serial exams post one week for surveillance for restenosis. Restenosis was defined as a renal artery PSV ≥ 180cm/s on follow-up RDS. Association between renal artery PSV and restenosis was examined using proportional hazards regression.
RESULTS:
RA-PTAS was performed on 187 renal arteries in 151 patients during the study period. Patients without RDS within the first week post RA-PTAS or on follow-up and redo interventions were excluded. 100 RA-PTAS procedures in 91 patients (58% female; 11% non-white; mean age, 70 ± 9.8 years; mean pre RA-PTAS PSV, 272 ± 104cm/s) were included in the study. Mean follow-up time was 14.9 ± 10.8 months. Figure 1 shows product limit estimate of time to primary patency failure. There was no significant difference in the mean PSV pre RA-PTAS in those with and without recurrent stenosis on follow up (283 ± 94cm/s, 265 ± 109cm/s respectively; P=0.417) and PSV pre RA-PTAS was not predictive of recurrence in proportional hazards regression (HR, 1.116; 95% CI, 0.90-1.52; P=0.25). Within one week of RA-PTAS mean renal artery PSV differed significantly for renal arteries with and without recurrent stenosis (114 ± 27cm/s, 91 ± 34cm/s respectively; P<0.001). Proportional hazards regression analysis demonstrated increased PSV on first post RA-PTAS RDS as predictive of recurrent disease (hazard ratio [HR] for 30cm/s increase, 1.83; 95% confidence interval [CI], 1.35-2.47; P<.0001). There was no difference in pre-op to post-op change in PSV in those with and without recurrent stenosis on follow up (169 ± 102cm/s, 174 ± 116cm/s respectively; P= 0.80) nor was this predictive of time to recurrent stenosis (HR, 1.02; 95% CI, 0.76-1.36; P=0.91). Multivariable associations between preoperative factors, first post-op PSV and follow up restenosis were examined using forward stepwise variable selection. Only first post-op PSV (HR for 30cm/s increase, 1.89; 95% CI, 1.40-2.57; P<0.0001) and bilateral RA-PTAS (HR 0.19; 95% CI, 0.04-0.99; P=0.049) were selected as significant predictors. A receiver operating characteristic (ROC) curve was examined to assess ability of first week PSV after RA-PTAS to predict follow-up restenosis. Optimal renal artery PSV cut point was 107cm/s or greater to predict follow up restenosis. This value was associated with a sensitivity of 64.9%, specificity of 71.4%, and area under ROC curve of 70.4%.
CONCLUSIONS:
Early renal artery PSV within one week after RA-PTAS predicts recurrent RAS. Recurrent stenosis demonstrated no association with absolute elevation in PSV prior to RA-PTAS nor in the change in PSV after RA-PTAS. 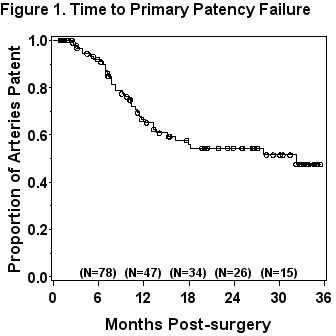
Back to Annual Meeting Program
