Back to Annual Meeting Program
Establishing Polymeric Transfection as a Non-Viral, Non-Toxic Method for Gene Therapy in the Prevention of Vascular Disease
Joshua D. Arnold, Deidra J.H. Mountain, Michael B. Freeman, Stacy S. Kirkpatrick, Scott L. Stevens, Mitchell H. Goldman, Oscar H. Grandas
University of Tennessee Graduate School of Medicine, Knoxville, TN
OBJECTIVE
Gene therapy shows promise in the treatment of vascular disease, but a formidable challenge has been delivery of genetic material in a safe and non-toxic way. Traditional transfection methods commonly used in the laboratory are poorly translatable in vivo, and there are currently no FDA-approved gene therapy products for clinical application. Biodegradable polymers, particularly poly(B-amino ester)s, have shown promise in their ability to deliver genetic material to a cell in a predictable and non-toxic way. However, the preponderance of early feasibility studies were tested and validated exclusively in stem cells. While stem cell derived therapies are promising in areas such as vascular tissue engineering and angiogenesis, dysfunctional regulation in differentiated cells is the primary source of pathogenesis. As such, gene therapy in these differentiated cell types would be prime targets for therapeutic gene modulation in the prevention of hyperplasia and restenosis. Here we aim to establish polymeric transfection as a feasible non-viral, non-toxic method for gene therapy in cells of vascular origin and in vascular tissue.
METHODS
Human aortic smooth muscle cells (HASMC) were transfected in vitro and rodent carotid artery segments were transfected ex vivo with poly(β-amino ester) polymers (StemFECT™) conjugated to Cy3-labeled GAPDH or negative control (NC) siRNA. Increasing polymer : siRNA ratios were tested for optimal transfection efficiency. At 48hrs post-transfection a live-dead stain was performed to confirm cell/vessel viability, conjugate endocytosis was confirmed by fluorescent microscopy, and GAPDH gene silencing was measured by qPCR normalized to 18S.
RESULTS
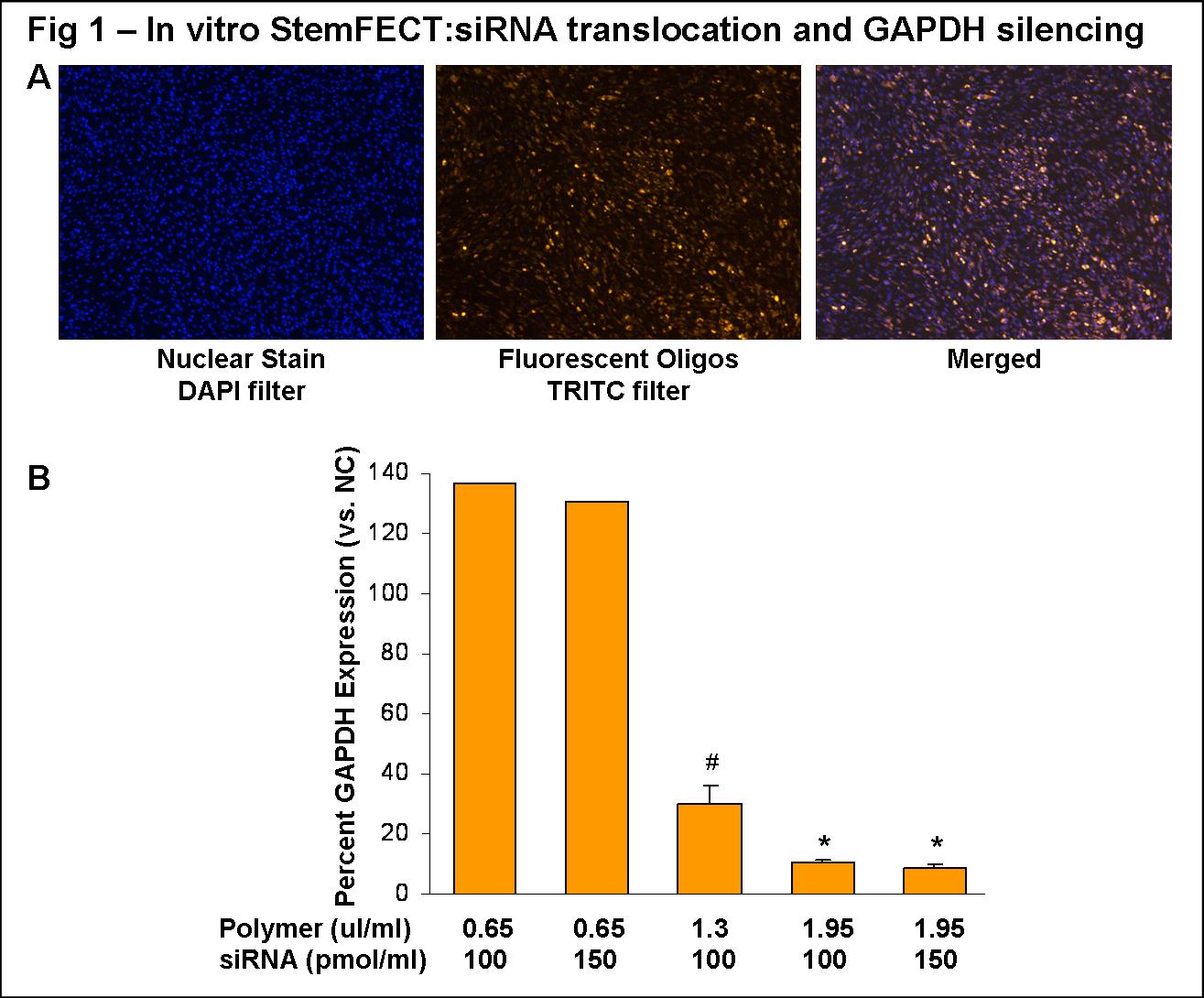
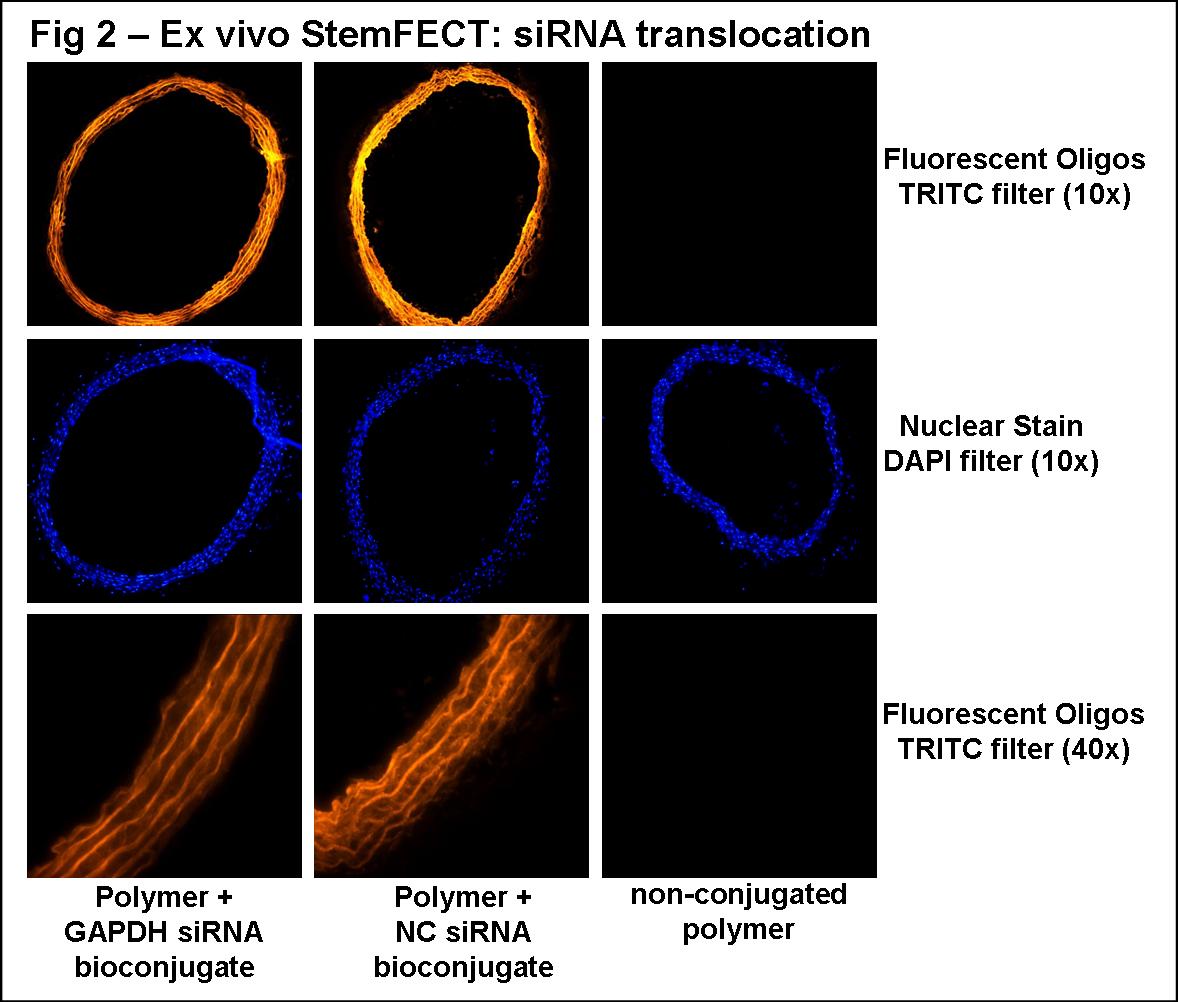
Cellular translocation of the polymeric bioconjugates in HASMC in vitro was visible using a 0.65µl polymer: 100pmol siRNA/ml conjugate (Fig 1A). Efficient gene silencing was achieved in a polymer concentration dependent manner, independent of increasing siRNA concentrations (Fig 1B). The most efficient in vitro GAPDH silencing was with a 1.95µl polymer: 100pmol siRNA/ml conjugate (10±1% expression vs. NC; n=5; *P<0.05 vs. NC and 1.3µl:100pmol conjugate). Using this bioconjugate ratio for vessel transfection ex vivo, translocation was visible in all cellular layers (Fig 2B; images representative of proximal, medial, and distal sections), and modest GAPDH silencing was observed (76±8% expression vs. NC; n=2).
ConclusionHASMCs and whole vessel segments were successfully transfected using polymeric bioconjugates. The significant gene silencing accomplished here establishes our proof of concept that polymeric transfection is a viable method for in vitro and ex vivo investigation in vascular cells. Future studies will expand on this method of gene therapy for in vivo transfection in animal models of vascular disease. Our long term goal is to deliver molecular inhibitors of genes thought to play a role in intimal hyperplasia, restenosis, and vessel graft failure. Polymeric transfection may translate to targeted genetic therapy for vascular disease.
Back to Annual Meeting Program
