Back to Annual Meeting Program
Characterization of the Lung Endothelial Cell Injury Response During Ischemic Acute Kidney Injury: The Role of TNF Signaling
Laura White1, Yan Cui1, Rachel J Santora1, Carolyn M Feltes-Shelak2, Heitham T Hassoun3
1The Methodist Hospital Department of Surgery and Research Institute, Houston, TX;2Legacy Emmanuel Children's Hospital, Portland, OR;3The Methodist Hospital Department of Surgery and Research Institute and The Methodist DeBakey Heart & Vascular Center, Houston, TX
Introduction: Despite advancements in renal replacement therapy, the mortality rate for acute kidney injury (AKI) remains unchanged over the past several decades, likely due to extra-renal organ dysfunction. Kidney ischemia-reperfusion injury (IRI) activates cellular and soluble mediators that stimulate the innate and adaptive immune systems and incite a distinct remote organ pulmonary pro-inflammatory and pro-apoptotic response. Ischemic AKI induces tumor necrosis factor 1 (TNFR1)-dependent lung apoptosis and results in lung microvascular barrier dysfunction in vivo. Here, we focused on kidney-lung crosstalk at the cellular level and hypothesized that endothelial cells (ECs), with their critical role in maintaining the semipermeable barrier, are important targets leading to disruption of microvascular permeability during ischemic AKI. In a series of parallel in vivo and in vitro experiments, we sought to characterize the pulmonary EC-specific transcriptional and structural response to kidney IRI and hypothesized that TNF-α/TNFR1 signaling inhibition would attenuate EC alterations during ischemic AKI.
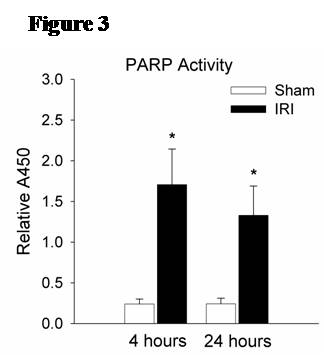
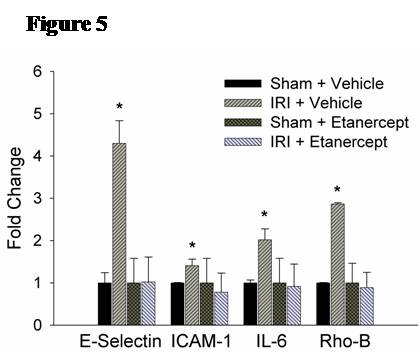
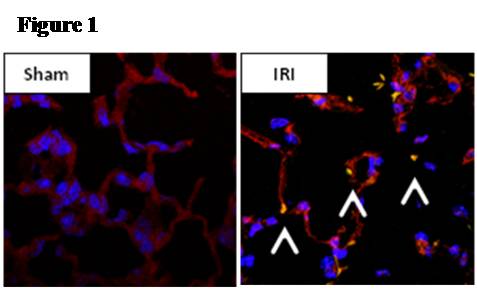
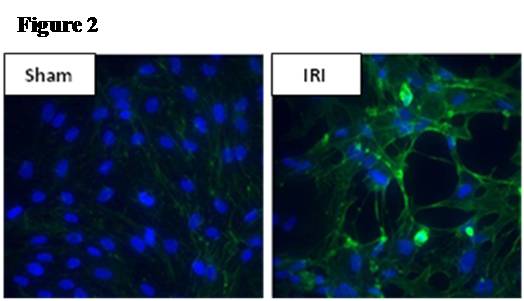
Methods: Male C57/BL6 mice or Sprague-Dawley rats underwent 60 minutes of bilateral kidney ischemia (IRI) or sham laparotomy (sham) and were sacrificed at 24 hours. In mice, co-localization studies with DAPI, TUNEL, and CD34 staining were performed, and lung microvascular ECs [CD45-/CD31+] were isolated with novel tissue digestion techniques followed by antibody-coupled magnetic bead sorting to 95% purity (by flow cytometry). Isolated ECs were analyzed with Apoptosis and EC Activation RT-PCR “SuperArrays” each containing 84 pathway-specific genes. In parallel, rat lung microvascular ECs (RLMVECs) grown to confluence were treated for 24 hours with serum from rats during IRI or sham, and assays included 1) DAPI/phalloidin staining to assess the actin cytoskeleton, 2) poly(ADP-ribose) polymerase(PARP) activity assay to measure apoptosis, and 3) RT-PCR to quantify transcription of TNF super-family and pro-inflammatory genes +/- pre-treatment with Etanercept .
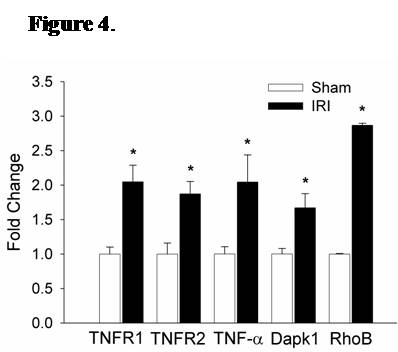
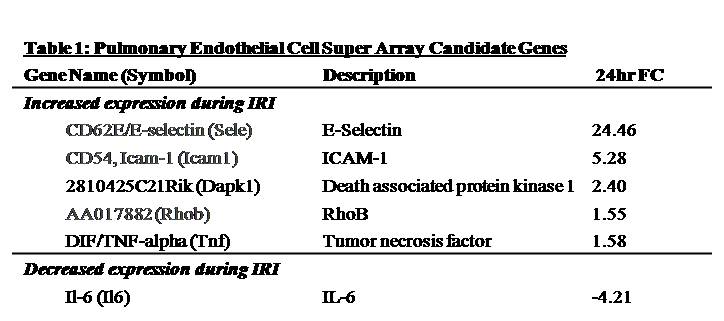
Results: Immunofluorescence micrographs stained with DAPI, TUNEL and CD34 co-localized, suggesting lung EC apoptosis in vivo during ischemic AKI. Isolated lung ECs exhibited significant changes during ischemic AKI in prominent genes identified by “SuperArray” analysis, including genes related to apoptosis (Dapk1, RhoB, Bcl10, FasL, Cflar), the TNF super-family (TNF-α, Tnfrsf10b[DR5], Trp53inp1, Trp63) and inflammation (E-Selectin, ICAM-1, IL-6). In vitro, RLMVECs treated with IRI serum displayed actin stress fiber formation and increased apoptosis at 4 and 24 hours compared to sham (0.24±0.06 vs. 1.71±0.44*; 0.24±0.07 vs. 1.33±0.36*). RLMVECs also demonstrated increased transcription of TNF super-family related genes during IRI, and the pro-inflammatory transcriptional response was attenuated by TNF-α/TNFR1 signaling inhibition with Etanercept. *p<0.05 vs. sham, n≥4/group.
Conclusions: Kidney-lung crosstalk during ischemic AKI is a complex pathophysiological process involving the activation of several key cellular and soluble factors that drive remote organ injury. Ischemic AKI produces changes in the pulmonary EC phenotype with the induction of actin cytoskeleton rearrangement, apoptosis, and a distinct pro-inflammatory and pro-apoptotic transcriptional response. Additionally, kidney IRI drives increased transcription of TNF super-family genes, and TNF-α/TNFR1 signaling inhibition attenuates the EC inflammatory response in vitro. Future endeavors to characterize the EC-specific response and effector mechanisms to remote organ injury during ischemic AKI may reveal potential therapeutic targets for kidney-lung crosstalk.
Back to Annual Meeting Program
