Back to Annual Meeting Program
Percutaneous Endovascular Aortic Aneurysm Repair (PEVAR): Results from the First Prospective, Multicenter Randomized Trial
Peter R Nelson
University of Florida College of Medicine, Gainesville, FL
BACKGROUND:The first randomized controlled trial was designed and conducted to assess the safety and effectiveness of totally percutaneous endovascular aortic aneurysm repair (PEVAR) using a 21Fr endovascular stent graft system and either an 8Fr or 10Fr suture-mediated closure system. A non-inferiority trail design was chosen to compare percutaneous access to standard open femoral exposure.
METHODS:Between 2010 and 2012, 20 US institutions participated in a prospective, Food and Drug Administration-approved randomized trial to evaluate the safety and effectiveness of percutaneous femoral artery access and closure using a ‘pre-close’ technique in conjunction with endovascular abdominal aortic aneurysm repair (EVAR). A total of 192 patients were enrolled, 41 in a non-randomized roll-in phase, and then 151 in the randomized phase where patients were allocated 2:1 to percutaneous closure [Group C] (N=101) or open femoral exposure [Group S] (N=50). PEVAR procedures were performed using either the 8Fr Perclose ProGlide [Group C1] (N=50) or the 10Fr Prostar XL [Group C2] (N=51) closure device. All EVAR procedures were performed with the 21Fr profile IntuiTrak System. Patients were screened using computed tomography with 3D reconstruction and independent physician review for anatomic suitability and adequate femoral artery anatomy for percutaneous access (e.g., absence of anterior wall or circumferential calcification, aneurysm, or extensive scarring). Primary Treatment Success was defined as procedural technical success and absence of adverse systemic and access-related vascular events at 30 days. Secondary clinical utility and procedural outcomes, ankle-brachial index, blood laboratory analyses, and quality of life were also evaluated with continuing follow-up to six months.
RESULTS:Baseline characteristics were similar among all groups. EVAR technical success was 100%, 100%, and 98% in Groups C1, C2, and S, respectively. Primary Treatment Success at one month was 88% (C1), 78% (C2), and 78% (S) with a one-sided Blackwelder’s test of non-inferiority yielding P=.0036 (C1 vs. S) and P=.1021 (C2 vs. S). Secondary outcomes procedurally to within one month are shown in the table below. PEVAR [Group C] compared favorably with respect to time to hemostasis, anesthesia time, total procedure time, analgesic use, ipsilateral groin pain, blood transfusion requirement and quality of life metrics. Final six-month follow-up is ongoing.
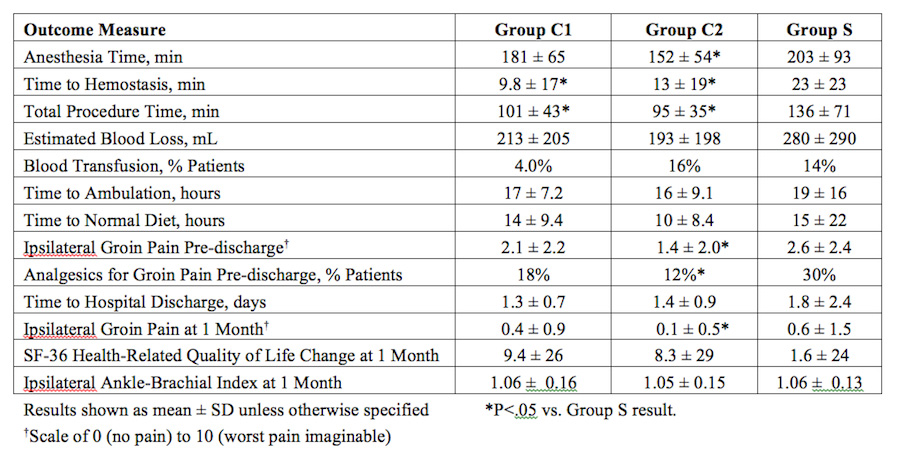
CONCLUSIONS:Among trained operators and patients with suitable femoral artery anatomy, a totally percutaneous approach to EVAR is safe, with minimal access-related complications. The ProGlide suture-mediated device specifically performed non-inferior to standard open femoral exposure. Training, experience, and careful application of the ‘pre-close’ technique is of paramount importance in ensuring successful, sustainable outcomes.
Back to Annual Meeting Program

