Liposomal Nanocarriers Designed for Sub-Endothelial Matrix Targeting Under Vascular Sheer Stress
Lauren B Grimsley, Richard K Fisher, III, Raymond A Dieter, III, Joshua D Arnold, M Ryan Buckley, Michael M McNally, Scott L Stevens, Michael B Freeman, Deidra J.H. Mountain, Oscar H Grandas
University of Tennessee Graduate School of Medicine, Knoxville, TN
INTRODUCTION: Vascular interventions inherently result in disruption of the tunica intima and exposure of sub-endothelial matrix proteins. Spatially controlled nanoparticles designed to co-localize to these exposed matrices could provide a targeted drug delivery system aimed at inhibiting dysfunctional vascular remodeling and improving intervention outcomes. We have previously reported the development of a surface-modified liposomal platform designed to preferentially bind collagen type IV, an abundant sub-endothelial matrix protein, in a static in vitro environment. Here we present our progress in this discovery-driven liposomal nanocarrier system by validating its collagen IV binding affinity under simulated physiological and pathological vascular flow conditions.
METHODS: Non-targeting PEGylated liposomes (PLP) were formed with bulk DOPC-PEG + 30mol% cholesterol + 0.1mol% Rhodamine-DOPE. Collagen-targeting liposomes (CT-PLP) were formed by reacting DSPE-PEG-DBCO to previously established collagen binding peptides (CBP), via copper-free click chemistry, and inserting 5mol% CBP-modified lipids to base PLPs at lipid hydration. Collagen IV matrices were dried at 3ug/cm2, and hemodynamic liposome binding was assayed by live fluorescence microscopy over 60min simulated flow at 5-115 dynes-s/cm2 using a closed parallel-plate flow chamber. Liposome binding was quantified by mean fluorescent intensity of bound Rhodamine-labeled lipid normalized to background.
RESULTS: Under continuous flow, CT-PLPs demonstrated increased binding to collagen matrices over time at all tested sheer stress conditions (Fig1A). After 60min flow, CT-PLPs demonstrated remarkable binding under all conditions while non-targeted PLP control binding was negligible (Fig1B).
CONCLUSIONS: CT-PLPs demonstrated an affinity for collagen IV binding under simulated hemodynamic flow at sheer stress conditions ranging from venous physiological to pathological flow and arterial physiological to pathological flow. CT-PLP nanocarriers established here show promise as the framework for a spatially controlled drug delivery platform for future application in targeted vascular therapeutics. Ongoing studies aim to demonstrate CT-PLP binding capacity in situ via ex vivo human saphenous vein and femoral artery perfusion under normal and elevated sheer stress. 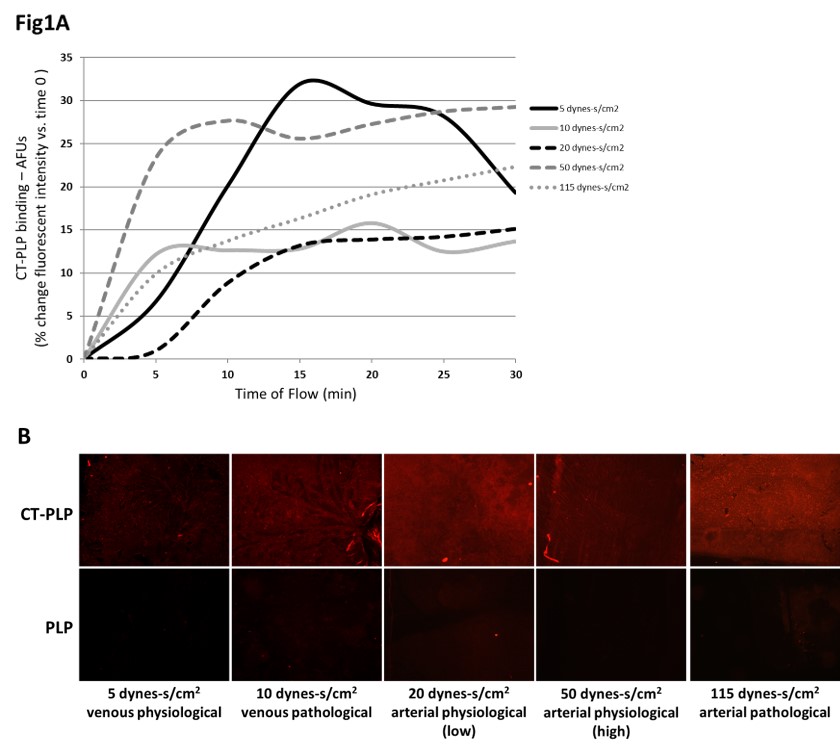
Back to 2019 Abstracts
