Back to 2016 Annual Meeting Program
A Novel Large Animal Model of Peripheral Arterial Disease
Chandler A Long1, Michael Sweet2, Tatiana Chadid1, Panagiotis Koutakis2, Traci Goodchild3, David Lefer1, Iraklis Pipinos4, George Casale1, W. Robert Taylor1, Luke Brewster1
1Emory University School of Medicine, Atlanta, GA;2Emory Healthcare, T3 laboratoies, Atlanta, GA;3Georgia Institute of Technology, Parker H. Petit Institute for Bioengineering and Bioscience;, Atlanta, GA;4emory healthcare, T3 laboratoies, Atlanta, GA
OBJECTIVE: Critical Limb Ischemia (CLI) is an insidious condition that carries a 25-40% risk of major amputation and an annual mortality rate of 20%. The condition is estimated to affect between five-hundred thousand to one million patients annually. Up to 10% of peripheral arterial disease (PAD) patients over the age of 50 will develop CLI within 5 years. Given the aging of our society and the increasing longevity of patients with cardiovascular disease, it is estimated that CLI will assume an even greater impact on societal health in the years to come. Prompt revascularization can serve as both life and limb saving. However, many CLI patients do not have traditional revascularization options. Therefore, it is important to develop novel therapies that promote vascular regeneration at the bench to translate to the bedside.
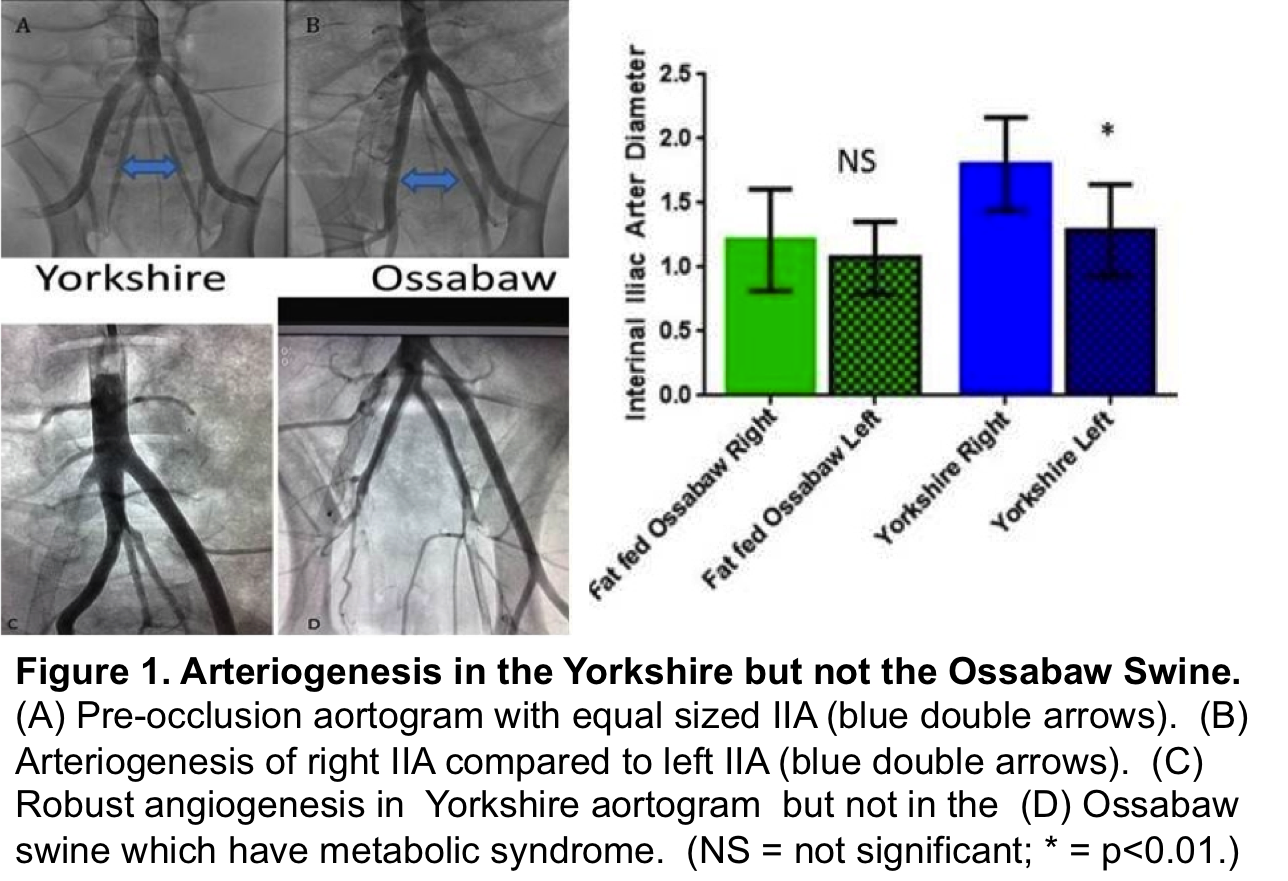
While many exciting ideas have been proposed, a limiting factor to clinical translation is the lack of a large animal model that shares fidelity to the muscular ischemia seen in PAD and/or CLI. Such models could be used to test the optimal medication dosages, delivery methods, and suitability of re-dosing strategies. Our objective in this study was to create and describe a novel and minimally invasive large animal model of PAD that shares similarities to the ischemic myopathy seen in ischemic human limbs. Here we test this model in healthy (Yorkshire) swine and compare the arteriogenic response to this model in a metabolic syndrome (fat fed Ossabaw) swine.
METHODS: For the two groups of Swine used in the study [8 Yorkshire swine and 8 Ossabaw swine], ipsilateral (right leg) occlusion of the external iliac artery and its collateral vessels were induced by means of endovascular occlusion and plug placement. Endovascular access to the infrarenal aorta was obtained through open exposure of the left common carotid artery. After arteriograms were completed, a Gore Viabahn stent © was placed from the ostia of the external iliac to the common femoral artery. An Amplatzer © vascular plug was then deployed within the stent. This achieved prevention of recanalization by collateral arteries with the covered stent and obstruction of inline flow with the vascular plug.
Arteriograms were performed at baseline and termination. These arteriograms were used to examine arteriogenic capacity of the ipsilateral internal iliac arteries and time-of-flight analyses from the aorta to the superficial femoral artery. Arteriogenic capacity was compared between groups of swine using pre and post-occlusion measurements of the right (ischemic) and left (non-manipulated) internal iliac arteries. Time-of-flight was measured directly from the initiation of contrast bolus in the distal aorta until time of contrast entering the common femoral artery. Measurements were compared right (occluded EIA) to left (normal EIA).
The Yorkshire swine underwent weekly hindlimb blood pressure and femoral artery duplex ultrasounds. In addition, videos analysis of the Yorkshire swine’s gait was captured weekly in the animal vivarium to demonstrate the functional impact of ischemia. Yorkshire animals were terminated at 5 weeks (n=5) and at 6 weeks (n=3) postoperatively. Gastrocnemius and plantaris muscle samples were analyzed on a blind basis and quantitatively scored to assess the degree of skeletal muscle ischemic myopathy
RESULTS
Perioperative events. One Yorkshire pig died prior to experimental intervention due to restrictive pericarditis diagnosed on necroscopy. This animal was replaced with a subsequent animal for a total of 8 Yorkshire pigs that completed the study.
Arteriogenesis. There was robust arteriogenesis of the ipsilateral Yorkshire IIA. This was not present in the fat-fed Ossabaw swine. (Figure 1)
Time of flight flow and wall shear stress analyses. At time of acute occlusion, there was no flow identified in the right common femoral and superficial femoral arteries. At terminal arteriogram (5 weeks post-ischemia), there was reconstitution of these arteries by retrograde geniculate collateral flow. However as expected, there was significant delay in filling of the femoral arteries on the right compared to left side [2.8 seconds vs. 0.3 seconds; P=.003]. In addition the reversal of flow with retrograde filling of these arteries from the geniculate system signifies an oscillatory index of 0.5.
Hindlimb index (HLI). The ischemic hindlimb occlusion pressure was significantly depressed in the right versus left leg out to 6 weeks [SBP 31±21 versus 83±15; P=.0007]. The hindlimb index compared right to left hindlimb blood pressure measurements as a surrogate to the ankle brachial index. The HLI plateaued from weeks 2-5 (~0.4) with a slight uptick in week six. (Figure 2 A)
US velocities. The peak systolic velocity of the common femoral artery was severely depressed on the right compared to left throughout the time course of this study [P<.001 at all time points]. The greatest increase in velocity occurred between 1 and 2 weeks post occlusion. Velocities in the right hindlimb disappear and slowly recover over 5 weeks to 38% of their baseline values. (Figure 2 B) These velocities directly correlated to the gain in HLI values over time. Interestingly the end diastolic velocity (EDV) was not significantly different between groups. (data not shown)
Functional deficit. The functional deficit in the dorsiflexion of the right hoof was measured by weekly videotaping of gait. Although the animals favored their right hoof, all animals recovered normal gait by post-occlusion day 21. Animals were noted to have an initial deficit that recovered by week 3.
Ischemic myopathy. To date only the Ossabaw swine plantaris and gastrocnemius muscles have been analyzed. These were examined in a blinded fashion and scored semi-quantitatively by an ischemic myopathy score. The ischemic limb’s muscle had significantly more severe ischemia [Score of 2±1 vs. 1 ±1; P=.00064].
DISCUSSION: We have introduced and characterized a novel porcine model of CLI using endovascular techniques that provides sustained ischemia out to six weeks by occluding inline flow through the external iliac artery and impedes the rapid arterial collateralization through the use of a covered stent. Furthermore this minimally invasive approach obviates the contamination of the extremity muscle specimens that occur with open arterial ligation models. These benefits uniquely position this model for testing of regenerative therapies.
We correlate the recovery of perfusion over time to the robust arteriogenesis of the internal iliac artery noted in the healthy Yorkshire pig. Interestingly, the fat fed Ossabaw failed to demonstrate the capacity for arteriogenesis and recovery witnessed in the Yorkshire. This observation is consistent with the clinical scenario seen in CLI patients with metabolic syndrome, where arteriogenesis is limited.
The current limitations of this model include the natural progression of perfusion in the Yorkshire swine over time. It is likely that Yorkshire swine will behave similar to patients with iliac artery occlusion and stabilize with an ABI of ~0.8. In addition we are currently in the process of scoring the Yorkshire swine musculature. It will be important to demonstrate similar ischemic myopathy in the Yorkshire as we identified in the Ossabaw.
There are a number of future directions with this model that we are pursuing. First since patients with iliac occlusion can have their underlying ischemia unmasked with exercise, validation of this phenomena in the animal model may be useful to exercise testing. Second, this model is being explored with perfusion imaging to correlate the histologic findings with non-invasive imaging. Finally we propose that this model is well suited to test regenerative therapies, particularly between weeks 2 and 5.

Back to 2016 Annual Meeting Program
