Clopidogrel Resistance is Common in Patients Undergoing Vascular and Coronary Interventions
Thomas N. Hawken, Adam M. Berenson, Jennifer Klopfenstein, Charles C. Leithead, Clayton J. Brinster, Waldemar C. Sternbergh, III, Samuel R. Money, Stephen Ramee, Daniel Fort, Hernan A. Bazan
Ochsner Medical Foundation, New Orleans, LA
Introduction:‘Clopidogrel resistance’ is a term used to describe the clinical scenario wherein clinicians are unable to achieve the desired anti-thrombotic effect with the anti-platelet medicine, clopidogrel. The problem is also defined throughout the literature as ‘heightened platelet reactivity’ (HPR) while on clopidogrel therapy, where platelet activity is not inhibited by clopidogrel despite adequate dosing. Persistent platelet activity can lead to platelet aggregation and, potentially, a clinical thrombotic event. A recent meta-analysis described ‘clopidogrel resistance’ or poor biological response to clopidogrel with a wide range between 16-50% (1). However, the included studies are limited by different testing strategies and small patient series. To date, there are no large series that have examined the prevalence of HPR in a mixed cohort of vascular and coronary patients, and there is little data to offer guidance in the diagnosis and management of HPR for vascular surgery patients. Though the clinical significance of 'clopidogrel resistance' or HPR has not been evaluated in a randomized controlled trial, a recent report of 379 patients with symptomatic coronary disease treated by stenting found that patients with 'clopidogrel resistance' or HPR had significantly more cardiovascular events and death (2). Following certain vascular and coronary interventions, effective dual antiplatelet therapy (DAPT) is desired to maintain patency and avoid an acute thrombotic event. The potent antiplatelets agents clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brillinta) can be categorized into thienopyridine (e.g. clopidogrel, prasugrel) and non-thienopyridine agents (e.g. ticagrelor). All of these agents inhibit the function of the platelet cell surface receptor P2Y12, an adenosine diphosphate (ADP)-activated G-protein linked receptor. Blocking the P2Y12 receptor causes inhibition of platelet aggregation through a number of intracellular processes, resulting in the desired clinical platelet anti-thrombotic effect. Clopidogrel is a pro-drug that is metabolized into its active form by the cytochrome P450 system (specifically CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4/5). The active clopidogrel metabolite irreversibly blocks the P2Y12 ADP receptor, thereby reducing platelet aggregation. There are many proposed mechanisms for HPR, including genetic and co-ingestion of known inhibitors of the CYP450 system (3, 4). Verify Now-P2Y12 (Accumetrics, Inc.; San Diego, CA) is a commercial rapid platelet-function assay designed to measure the effects of clopidogrel on the P2Y12 receptor. Verify Now-P2Y12 assay results are expressed in P2Y12 reaction units (PRU). This test can usually be performed in less than 2 hours and is inexpensive, costing approximately in our health system. A PRU value > 200 while on clopidogrel indicates platelet aggregation is not inhibited by clopidogrel, suggesting that an alternative antiplatelet agent, such as prasugrel or ticagrelor, should be considered in addition to aspirin if DAPT is required for a particular patient. Despite numerous reports of HPR(1-3, 5, 6) , no randomized controlled trials exist to establish the appropriate indications for testing and assessment of clopidogrel resistance prior to percutaneous coronary interventions (PCIs) (7) or peripheral vascular interventions (8). A prospective study of 100 patients undergoing infrainguinal percutaneous transluminal arterial angioplasty or stenting found that clopidogrel resistance or HPR was associated with markedly increased adverse clinical events in patients undergoing these peripheral endovascular procedures(6). We aimed to identify the prevalence of ‘clopidogrel resistance’ or HPR in patients undergoing vascular and coronary interventions in a large, tertiary health system.
Methods: In our health system, the diagnostic test VerifyNow P2Y12 became available in October 2014. We identified all patients in our large integrated healthcare system with a measured PRU from 10/30/14 to 12/31/19. We identified patients who underwent an endovascular intervention or percutaneous coronary intervention during that time using CPT codes. We then placed patients into cohorts (peripheral vascular and/or coronary intervention) based on CPT code and PRU value. The peripheral vascular interventions (with corresponding CPT codes) included carotid artery intervention (37215, 37216, 37217, 37218), iliac/femoral/tibial/popliteal/peroneal intervention (37221, 37223, 37226, 37227, 37230, 37231, 37234, 37235), mesenteric intervention (37236, 37237), intracranial intervention (61635), extracranial vertebral intervention (0075T, 0076T), and venous intervention (36903, 36909, 37238, 37239). We also identified CPT codes for coronary interventions (92928, 92929, 92933, 92934, 92938, 92941, 92943, 92944). Demographic and comorbidity data were collected from review of our electronic health record. Prevalence of HPR was calculated in each of these cohorts. Fischer’s exact test was used to determine if relationships in the data were statistically significant.
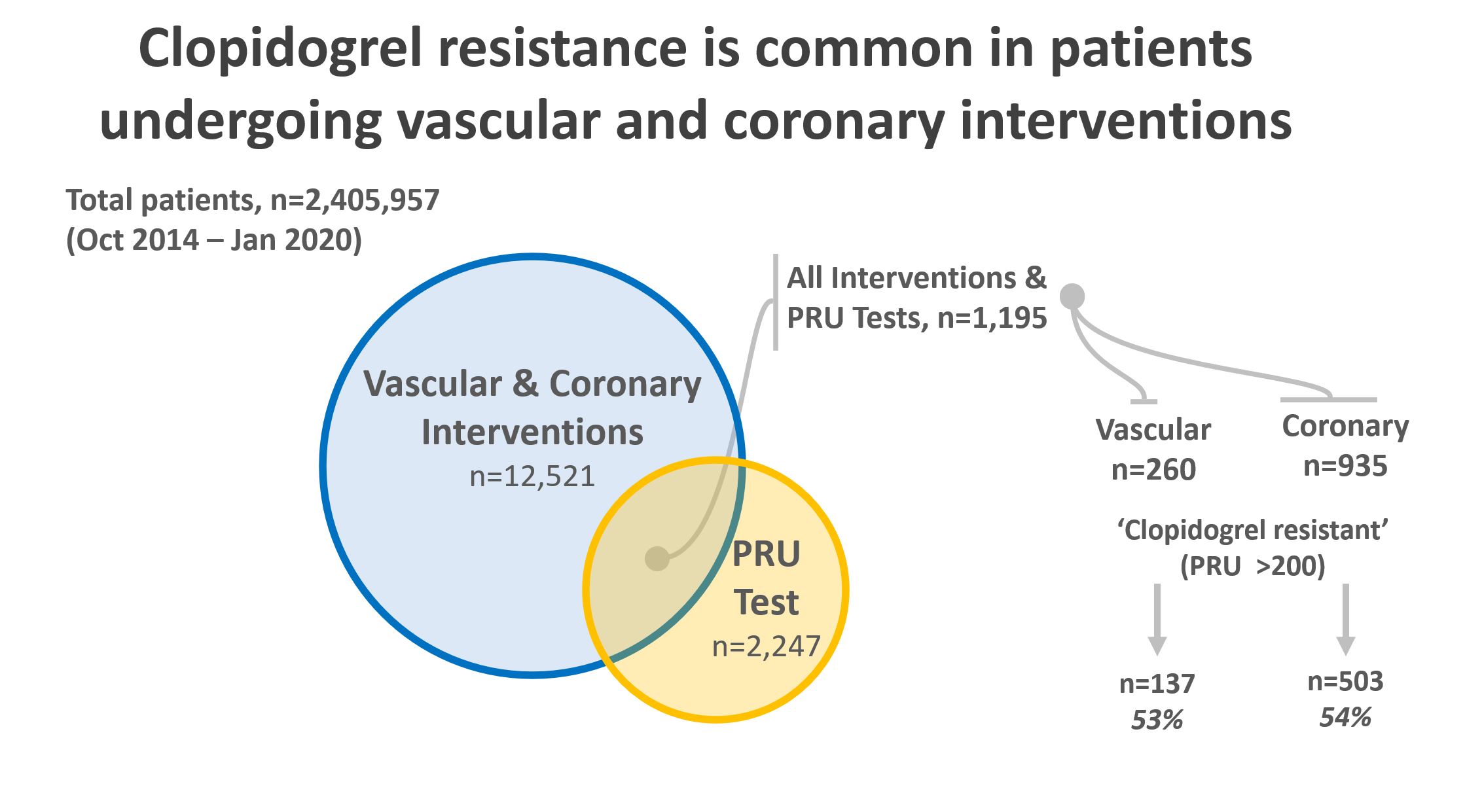
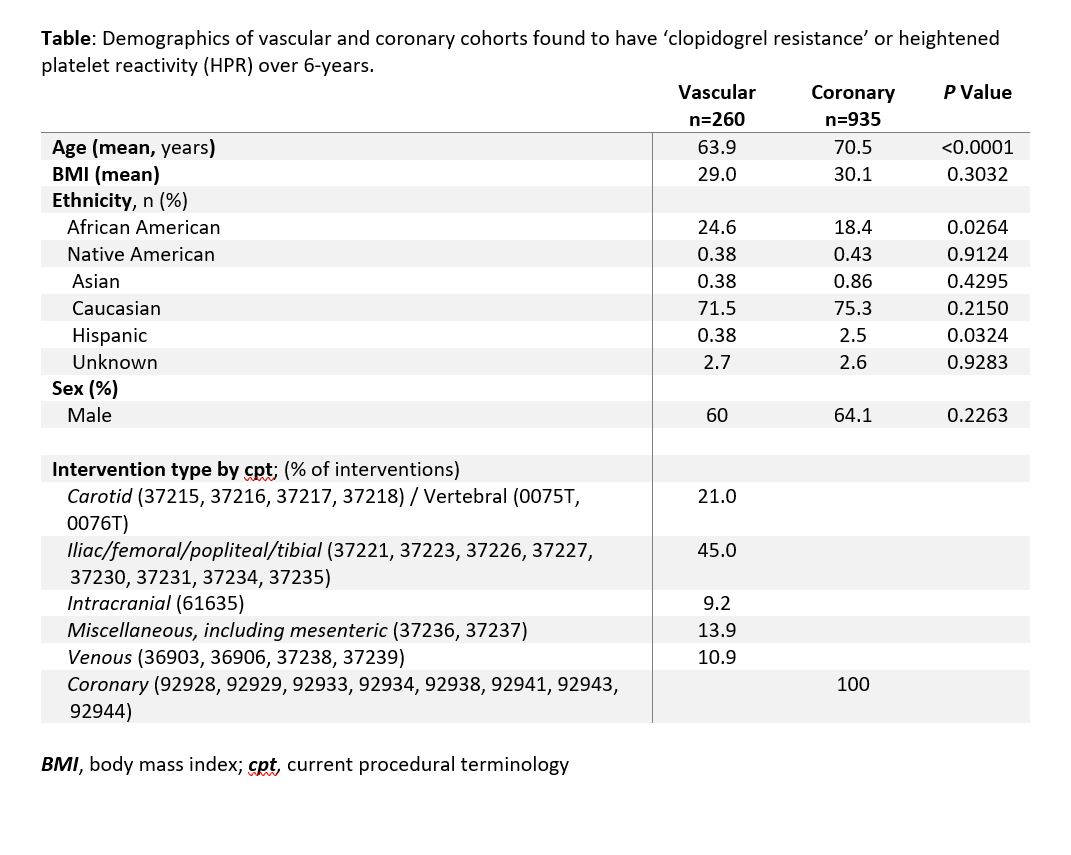
Results: From an initial cohort of 2,405,957 patients over a six-year period, 12,521 patients were identified who underwent endovascular or percutaneous coronary interventions. Of these, 2,247 patients had a PRU measurement during the study period. 1,195 patients had both an endovascular procedure and a PRU measurement (Figure). Of these, 935 were coronary interventions and 260 were peripheral vascular interventions. In the coronary cohort, 503 out of 935 patients had a measured PRU > 200, giving HPR prevalence of 54%. In the peripheral vascular cohort, 137 out of 260 had a PRU > 200, giving HPR prevalence of 53% (Table). There was no statistically significant difference in the prevalence of HPR between the two cohorts (p = 0.78). Patients undergoing coronary intervention were older than those undergoing vascular intervention (70.5 vs. 63.9 years, P <0.0001). More African Americans underwent vascular intervention (24.6% vs 18.4%, P =0.026); more Hispanic people (2.5% vs. 0.38%, P = 0.032) underwent coronary intervention. Iliac and infra-inguinal interventions (45.0%) were most common in the vascular cohort, followed by cerebrovascular (21.0%), and miscellaneous - including mesenteric interventions (13.9%). Evaluating patients for HPR was both inexpensive, costing , and rapid, assessed in less than 2 hours. For outpatient prescriptions, Medicare beneficiaries pay on average C:\inetpub\wwwroot\WebsiteHosting\SAVS\website\meeting\abstracts\2021\13.cgi-19 for clopidogrel, -139 for prasugrel, and -423 for ticagrelor (pricing information from goodrx.com, last accessed 8/28/20).
Discussion:Our data show that the prevalence of ‘clopidogrel resistance’ or HPR is at the high end of previously published ranges at 53% to 54%. Moreover, this high prevalence is noted in patients undergoing both coronary and vascular interventions in similar proportions. These results suggest that clopidogrel resistance, or HPR, is a more common problem in the vascular patient population than previously appreciated. Given reported data from small series suggesting a clinical benefit for clopidogrel resistance or HPR assessment in both coronary(2) and vascular(6)interventions, a point-of-care clopidogrel test prior to intervention might be useful in individualizing antiplatelet therapy to attain superior clinical results. Though current guidelines (7) for cardiology patients recommend against the routine use of platelet function testing, these guidelines do not expand on when to implement the selective use of such assays into decision making for personalized treatment approaches. Inter-individual response variability is a well-known phenomenon in clopidogrel therapy and is attributed to multiple causes, with some studies noting >70% of variability to be heritable (6). It has been estimated that individual genotypes, specifically the CYP2C19*2 loss of function mutation, may account for up to 12% of the variation in response to clopidogrel, while demographics and clinical factors likely contribute the large majority of clopidogrel resistance or HPR (4). Moreover, impaired enteric absorption, drug-drug interaction, and environmental influences may also play a role in decreased resistance (6). Many vascular beds do not tolerate treatment failure, including the cerebrovascular, coronary, mesenteric, and infra-inguinal arteries. While more expensive than clopidogrel, prasugrel and ticagrelor offer alternative DAPT for patients with HPR and in need of effective antiplatelet therapy. Our study is limited by its retrospective nature and lack of randomization of patients. Despite these limitations, our data suggest 'clopidogrel resistance' or HPR is an under-appreciated problem for vascular surgery patients. Future randomized prospective study will be needed to determine the effect 'clopidogrel resistance' or HPR has on patient outcomes.
References:1. N. Mallouk et al., Prevalence of poor biological response to clopidogrel: a systematic review. Thrombosis and haemostasis107, 494-506 (2012).2. T. Geisler et al., Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur Heart J 27, 2420-2425 (2006).3. A. Gonzalez et al., Effect of CYP2C19 Polymorphisms on the Platelet Response to Clopidogrel and Influence on the Effect of High Versus Standard Dose Clopidogrel in Carotid Artery Stenting. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 51, 175-186 (2016).4. L. Bonello et al., Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. Journal of the American College of Cardiology 56, 1630-1636 (2010).5. L. Bonello et al., Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. Journal of the American College of Cardiology 56, 919-933 (2010).6. S. Spiliopoulos et al., Platelet responsiveness to clopidogrel treatment after peripheral endovascular procedures: the PRECLOP study: clinical impact and optimal cutoff value of on-treatment high platelet reactivity. Journal of the American College of Cardiology 61, 2428-2434 (2013).7. D. Sibbing et al., Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc Interv 12, 1521-1537 (2019).8. M. Guirgis, P. Thompson, S. Jansen, Review of aspirin and clopidogrel resistance in peripheral arterial disease. Journal of vascular surgery 66, 1576-1586 (2017).
Back to 2021 Abstracts