Evaluation of an In Vivo Rat Carotid Artery Interposition Study to Assess Functionalized Tissue Engineered Vascular Scaffolds
Lauren N. West-Livingston1, Young Min Ju2, Huseyin Karagoz2, Fatih Zor2, Vijay Gorantla2, Randolph L. Geary3, Gabriela Velazquez-Ramirez3, Anthony Atala2, Sang Jin Lee2
1Wake Forest School of Medicine Department of Molecular Medicine and Translational Science, Winston-Salem, NC, 2Wake Forest Institute for Regenerative Medicine, Winston-Salem, NC, 3Wake Forest Baptist Health Department of Vascular and Endovascular Surgery, Winston-Salem, NC
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death globally, and represents a collection of often fatal ailments that could be addressed with preventative care.1,2 In advanced cases of CVD, surgical intervention is frequently utilized to improve or restore perfusion.3-5 For many vascular interventions, autologous vessels are the preferred conduit for bypasses and repairs.6 In cases where autologous vessels are unavailable, synthetic vessels are among the most popular alternatives.6 Synthetic grafts, however, often have challenges maintaining patency in the setting of small-diameter vessels, and require intervention or replacement relatively soon after intervention.6-8Tissue engineered vascular grafts (TEVGs) are a lucrative substitute to synthetic conduits. Recently, advancements in the field of in situ tissue engineering offer novel approaches to expediting the process of cellularizing scaffolds.9 Previous research has established that for successful small-diameter vascular grafts, the presence of endothelial cells (ECs) on the luminal surface and smooth muscle cells (SMCs) in the vessel wall contribute to the patency and anti-thrombotic properties of the graft.9 In order to examine an acellular TEVG in a physiological environment, a preclinical animal model ideal, as it allows assessment under physiological pressure and flow.10,11This study aimed to analyze the structural stability and patency of small-diameter electrospun vascular scaffolds in a rat model. The acellular scaffolds were modified with heparin to prevent thrombosis, and implanted as carotid artery interposition grafts. This preliminary animal model sought to establish time points for sacrifice to observe endothelialization and smooth muscle growth, as well as safety of an acellular scaffold for in vivo use.
2. METHODS
2.1. Scaffold Fabrication and Characterization
Fibrous scaffolds were fabricated by previously optimized electrospinning techniques.12-16 A 5% weight 1:1 PCL/collagen solution was prepared and scaffolds were fabricated through the electrospinning of the solution onto a 1.0 mm diameter cylindrical mandrel. Scaffolds were crosslinked, conjugated with heparin, and preserved in dI H2O at 4°C for no more than 24 h before use. Prior to implantation, scaffolds were freeze-dried, lyophilized, and sterilized. Cross-sections of the heparin-conjugated scaffolds were imaged via scanning electron microscope (SEM). The average wall thickness of scaffolds was assessed in triplicate.
2.2. In vivo Implantation
Six male Sprague Dawley rats, approximately 8 weeks old and/or 250 g, received scaffolds conjugated with heparin only. Two rats were sacrificed after each time point of 1 week, 3 weeks, and 8 weeks. Institutional Animal Care and Use Committee and NIH guidelines were followed for the care and utilization of all animal subjects.Animals were placed under general anesthesia and the right carotid artery was isolated. Approximately 2 cm of the artery was excised, and the experimental scaffolds were anastomosed in an end-to-end fashion with non-absorbable sutures. Suturing was performed by making three equally spaced interrupted sutures, with continuous running suture in between each interrupted suture. At the aforementioned time points, the implants were assessed for patency, retrieved, and then explanted, fixed and processed. The contralateral native carotid was also retrieved and served as the control condition.
2.3. Histological and Statistical Analysis
Retrieved scaffolds were fixed and prepared as slides. Cartilage and smooth muscle were examined through hematoxylin and eosin (HE) staining and Masson’s trichrome staining (MTS). The constructs were examined with an immunohistochemical stain (IHC) using anti-vonWillebrand factor (vWF) antibody to identify ECs and α-smooth muscle actin (SMA) antibody to identify SMCs. SPSS statistical software was used for data management and statistical evaluations. For continuous outcomes, the groups were compared using independent t-tests and one-way ANOVA with Bonferroni post hoc tests. Significance was considered at p < 0.05. Continuous data are reported as mean ± standard deviation.
3. RESULTS
3.1. Scaffold Characterization
SEM demonstrated that the electrospinning technique was replicable and produced comparable results between trials, and yielded scaffolds with mean wall thicknesses of 139.88 ± 3.95 µm (N = 9), 139.41 ± 3.46 µm (N = 9), and 140.58 ± 6.21 µm (N = 9), with no significant difference between any scaffolds (Fig. 1).
3.2. Rat Carotid Arterial Interposition Model
The scaffolds demonstrated similarity in inner diameter and wall thickness with the native vessel (Fig. 2). Post-surgical assessment of the anastomoses showed no evidence of leakage. The animals survived with no deficits at all time points, and the grafts demonstrated patency in 1-week and 3-week subjects demonstrated patency prior to explantation.
3.3. Histological Analysis
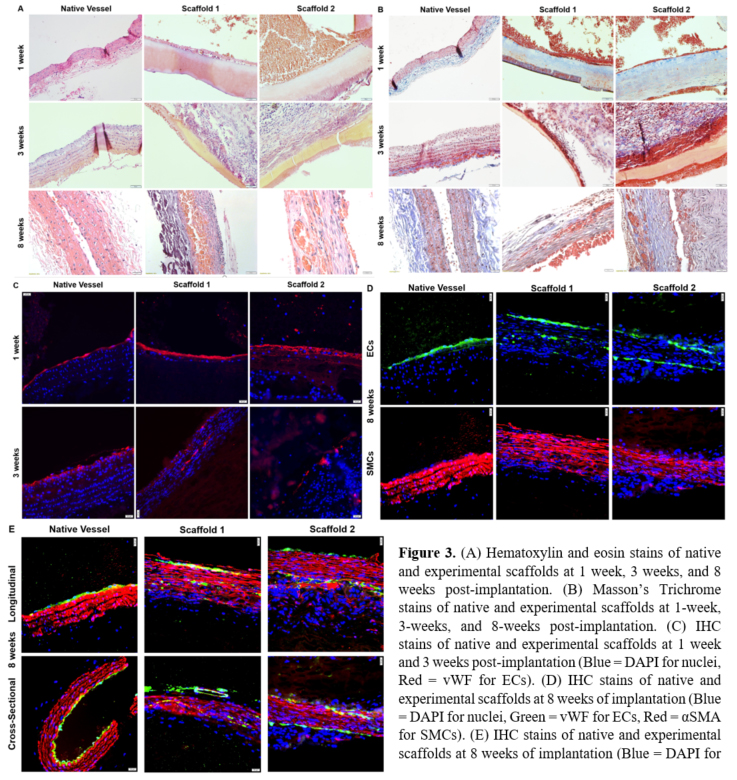
Both the 1-week and 3-week time points demonstrated deposition of tissue onto the luminal surface, with HE demonstrating nuclei and MTS ruling out collagenous tissue. At both time points,
the IHC did not indicate SMC growth on the scaffold, but indicated some degree of endothelialization on the luminal surface. Notably, the luminal layer at the 3-week time point appeared to be thicker than the endothelial monolayer of the native vessel. Though the 3-week scaffold showed discontinuous endothelialization, the gross appearance of the stain was more consistent with the pattern of the monolayer seen in the native vessel compared to 1-week results. Additionally, organization of nuclei indicated by DAPI staining at 3 weeks is analogous to the native vessel.At the 8-week time point, cellularization was observed on the luminal surface, as well as within the graft wall. Patency was compromised in the scaffolds at 8 weeks prior to explantation. Again, the HE stain and MTS demonstrated that nuclei were deposited onto the vascular lumen in a fashion that was thicker than in the native vessel, and the outermost layer in Scaffold 2 was grossly similar the endothelial monolayer observed in the native tissue. The IHC showed positive expression of both vWF and α-SMA, indicating a developing smooth muscle layer within the scaffold. While the SMCs in the experimental scaffold appeared less organized than those in native tissue, the DAPI-stained nuclei appeared to be more consistent with native organization (Fig. 3).
4. CONCLUSION
The experiments examining the use of acellular electrospun scaffolds in a rat carotid arterial interposition model suggest the described scaffold fabrication approach, as well as the employed surgical technique, are appropriate for this setting. Furthermore, the time points of 1 week and 3 weeks are suitable for examining the formation of an endothelial monolayer on the surface of the implanted graft. Longer time points will be needed to assess smooth muscle tissue
formation in scaffolds. Most importantly, time points between 3 and 8 weeks will be needed to further investigate the compromise of patency in this model at that time.
ACKNOWLEDGEMENTS
This work was supported by the Army, Navy, NIH, Air Force, VA, and Health Affairs to support the AFIRM II effort under Award No. W81XWH-14-2-0003. The U.S. Army Medical Research Acquisition Activity is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
REFERENCES
1.Association AH. Cardiovascular disease: A costly burden for America projections through 2035. In:2017.2.World Health Organization. Cardiovascular diseases (CVDs) [Fact Sheet]. In:2017.3.Starr JE. Surgical Bypass. 2017. Accessed November 4, 2017.4.National Heart Lung and Blood Institute. How Is Heart Disease Treated? 2014; https://www.nhlbi.nih.gov/health/health-topics/topics/hdw/treatment#. Accessed November 4, 2017.5.American Heart Association. Cardiac Procedures and Surgeries. 2017; http://www.heart.org/HEARTORG/Conditions/HeartAttack/PreventionTreatmentofHeartAttack/Cardiac-Procedures-and-Surgeries_UCM_303939_Article.jsp#.WgnpAVuPKUl. Accessed November 4, 2017.6.Pashneh-Tala S, MacNeil S, Claeyssens F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue engineering Part B, Reviews. 2015.7.Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. The Journal of Thoracic and Cardiovascular Surgery. 2010;139(2):431-436.e432.8.Wang L, Hu J, Sorek CE, Chen EY, Ma PX, Yang B. Fabrication of tissue-engineered vascular grafts with stem cells and stem cell-derived vascular cells. Expert Opinion on Biological Therapy. 2016;16(3):317-330.9.Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2014;6(1):61-76.10.Piedrahita JA, Williams JK. Animal Models in Tissue Engineering. Part I. Tissue Engineering Part C: Methods. 2017;23(11):641-642.11.Wissing TB, Bonito V, Bouten CVC, Smits AIPM. Biomaterial-driven in situ cardiovascular tissue engineering—a multi-disciplinary perspective. npj Regenerative Medicine. 2017;2(1):18.
12.Ju YM, Choi JS, Atala A, Yoo JJ, Lee SJ. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials. 2010;31(15):4313-4321.13.Lee SJ, Liu J, Oh SH, Soker S, Atala A, Yoo JJ. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials. 2008;29(19):2891-2898.14.Lee SJ, Yoo JJ, Lim GJ, Atala A, Stitzel J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. Journal of biomedical materials research Part A. 2007;83(4):999-1008.15.Lee J, Yoo JJ, Atala A, Lee SJ. Controlled heparin conjugation on electrospun poly(ε-caprolactone)/gelatin fibers for morphology-dependent protein delivery and enhanced cellular affinity. Acta biomaterialia. 2012;8(7):2549-2558.16.Ju YM, Ahn H, Arenas-Herrera J, et al. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta biomaterialia. 2017;59:58-67.
Back to 2021 Abstracts