Endograft exclusion of the false lumen restores local hemodynamics in a model of Type B aortic dissection
Joav Birjiniuk1, John Oshinski1, David Ku2, Ravi Veeraswamy3
1Emory University School of Medicine, Atlanta, GA;2Georgia Institute of Technology, Atlanta, GA;3Medical University of South Carolina, Charleston, SC
Introduction
The role of endovascular intervention in Type B dissection on aortic hemodynamics is poorly understood. While a stent graft may be indicated for patients facing immediate malperfusion or aneurysmal degeneration, its benefit in patients with uncomplicated disease is unclear. To date, only two randomized controlled trials have been undertaken to study the effect of optimal medical management of uncomplicated dissection with and without adjunctive TEVAR. Despite showing improvements in true lumen caliber and overall aortic modeling, these studies do not definitively demonstrate improved mortality or event-free survival.
Endograft deployment in these patients has focused on exclusion of the primary entry tear in order to eliminate false lumen flow and obliterate this channel. In theory this strategy could restore normal aortic hemodynamics both locally and distally along the extent of the dissection, but has yet to be demonstrated by a hemodynamic study. As such, alternative methods, such as the PETTICOAT technique of distal bare metal stent extension, have been executed in order to ensure complete distal false lumen exclusion. The present study was performed to characterize the hemodynamics in a dissected aorta following entry tear exclusion with a single graft and with subsequent extension of the graft along a distal dissection. We hypothesize that endograft deployment restores aortic hemodynamics locally, but does not eliminate distal false lumen canalization.
Methods
Anatomically accurate silicone models of aortic dissection were fabricated using patient CT images and a double-layer casting method. An intimal flap was created along the lateral descending aortic wall. Entry and exit tears were incised along the flap creating a dissection 20 cm long. Additional fenestrations were added at 0.5 and 0.75 of this distance in order to mimic true dissections with multiple flow pathways. Four total (two each of 80 mm and 60 mm) Valiant thoracic stent grafts (Medtronic, Santa Rosa, CA) were deployed in succession to cover each tear and 60% of the residual false lumen segment prior to each subsequent experiment (Figure 1A-D).
Grafted aorta models were installed on a flow loop and pulsatile, physiologic flow was delivered at mean arterial pressure of 100 and 140 mm Hg. Full flow fields were acquired using four-dimensional phase contrast magnetic resonance (PCMR) imaging. Gross qualification of aortic flows was performed using dye injection and computed flow visualization, which allows for particle tracing using the acquired MR velocities.
Whole aortic contours were segmented and centerlines calculated in each. Quantitative analyses were performed at five planes taken along the dissection at locations 0, 25%, 50%, 75%, and 100% of the longitudinal extent of the intimal flap. Absolute flow rates were calculated by integrating velocities across individual luminal cross-sectional areas. Relative luminal flow rates were then calculated as the relative proportion of these absolute rates to their sum (total aortic flow rate). Maximal flow velocities at peak systole were identified in false lumen sections as flows in these regions were near 0 when grafted. Flow reversal in these sections was quantified via the Reverse Flow Index (RFI), calculated as in Figure 1I, with Qreverse being the total flow back to the heart, Qforward the total flow towards the systemic circulation, and T the length of the cardiac cycle (1 second). This quantity represents the amount of blood volume traveling retrograde from a given slice compared to the total amount of blood leaving the slice across the cardiac cycle. True lumen flow rates in grafted segments were compared to flow rates in aorta devoid of dissection using unpaired two-tailed Studentís t-test.
Points along the aortic contour, along with the previously derived centerlines, were used to compute wall shear stress using a finite difference method. This allowed for quantification of oscillatory shear index (OSI), a surrogate for pro-thrombotic shear stress regimes (Figure 1J), in which the numerator represents the overall shear directed against the forward flow direction, and the denominator the total shear at a given wall point. This quantity has been shown to correlate with regions of atherogenesis in both carotid artery and abdominal aorta. Shear maps were then created by unwrapping aortic contours along the centerline in order to improve gross visualization and comparison.
Results
In all experimental groups, complete false lumen obliteration was achieved in the grafted region. At these locations, neither intimal flap nor false lumen was identifiable. Analysis of cross-sectional slices through these locations revealed relatively parabolic flow profiles in the true (total) lumen, with representative maximal velocities of 30.4 +/- 8.4 cm/s in the case of single graft deployment. No significant difference was found between flow rates in the true lumen of these segments and undissected aorta (p>0.05).
Partially covered flap segments between the distal extent of endografts and subsequent fenestrations acted as blind pouches creating retrograde filling from the true lumen (Figure 2B). While a false lumen could be visualized in these sections, these areas of residual false lumen were characterized by low velocity flows (maximum velocity 5.8 +/- 2.7 cm/s in the single graft condition) with decreased flow rates approaching nil and elevated oscillatory shear (Figure 3). False lumen flow waveforms demonstrate low flow rate oscillating about 0 correlating with a high reverse flow index (greater than 20% in all cases). However, the true lumen in these regions maintained forward flow (all RFI less than 6%) and a parabolic velocity profile, with maximum velocities approaching 28.4 +/- 1.3 cm/s.
In the regions of the free-standing intimal flap (i.e. segments with no endograft present, with intimal flap possessing tears on two ends), false lumen flow persisted, similar to the absence of endograft (Figure 2C). While the false lumen possessed a crescent-like cross-section with a dominant true lumen in these regions, velocities were comparable to those seen in the un-stented case (maximum velocity 7.0 +/- 2.1 cm/s). This true-dominant configuration maintained the parabolic velocity profile seen more proximally, with peak luminal velocities of 30.3 +/- 3.2 cm/s.
As with un-grafted dissection, computed visualization and dye studies demonstrated vortices developing at distal tear sites free of graft with mixing of true and false lumen flows. However, unlike the former, vortices forming distal to graft sites were predominantly left-handed, indicating flow of true lumen flow towards the false lumen flow. With the addition of successive devices to the model, fewer regions of false lumen were visualized, with subsequent loss of flow data in these regions. Trends seen in the different regions (grafted, blind pouch, persistent dissection) were maintained in their respective areas as grafts were added. Blind pouch regions exhibiting high OSI (Figure 1E, purple arrow) were eliminated when covered by stent-graft (Figure 1F-H).
Discussion
As hypothesized, endografts were found to restore normal parabolic velocity profiles in the segments that were stented. In addition, complete obliteration of the false lumen was achieved wherever an endograft was deployed. Blind pouch segments resulted from bare dissection segments between distal extents of grafts and subsequent fenestrations, and exhibited low-velocity, reversed flows that are suspect for coagulative thrombosis. As evidenced by the elevated Reverse Flow Index, fluid entering into these pouches retrograde incurs energy losses that are likely to lead to stagnation and false lumen thrombosis.
However, distal segments of false lumen with adjoining fenestrations were found to contain normal velocity flows and shear rates, and may therefore be protected from thrombosis. Distal to the trailing edge of the endograft, fluid was seen to traverse transluminally at exit tear sites, leading to left-handed diastolic vortices about the anterior-posterior axis (i.e. oriented from true to false lumen at fenestration sites). These vortices are observed primarily in a direction opposite to those seen in the absence of stenting, and indicate the presence of local, transient pressure defects in the false lumen at fenestration sites.
This work suggests that endograft exclusion of the proximal entry tear with concomitant false lumen obliteration is essential in preventing filling and patency of the false lumen from central aortic fluid flow. Additionally, it restores normal aortic hemodynamics locally at the site of deployment. Furthermore, it may aid in inducing false lumen thrombosis in the newly created blind pouch, leading to further closure of the false lumen. However, given these results, it may be insufficient in eliminating unfavorable hemodynamics downstream of the initial site of deployment. In fact, the transluminal fluid shift observed indicates that without further endograft deployment, the true lumen may maintain canalization of the distal false lumen. Prompt, more aggressive intervention may induce false lumen elimination by stagnation throughout the false lumen of the dissection.

Figure 1. Configurations of sequential device deployments in aortic dissection model (A-D). Changes in oscillatory shear index seen with additive device deployment in four-tear dissections possessing single (E), two (F), three (G), and four (H) devices. Calculation of RFI (I) and OSI (J).
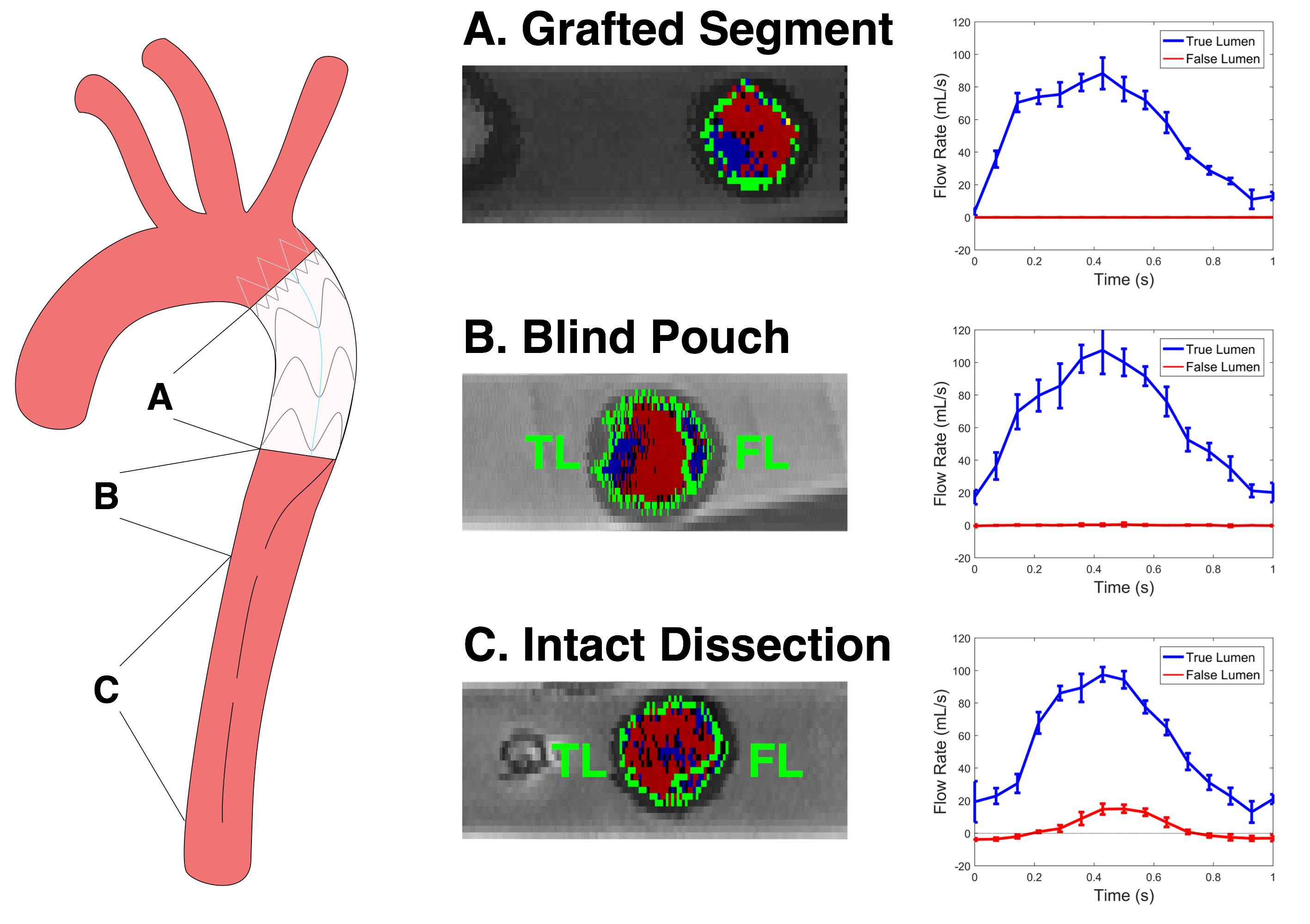
Figure 2. Demonstrative late diastolic axial velocity map images and true and false lumen waveforms in grafted dissection A, blind pouch region B, and persistent dissection C.
Back to 2019 Abstracts
