Development of a Multifunctionalized Vascular Scaffolding System to Induce In Situ Endothelialization
Lauren N West-Livingston1, Sang Jin Lee2, Young Min Ju2, Hyeongjin Lee2
1Wake Forest School of Medicine, Winston-Salem, NC;2Wake Forest Institute of Regenerative Medicine, Winston-Salem, NC
BACKGROUND – Vascular tissue engineered grafts are one of the most promising alternatives to small-diameter prosthetic grafts, which often require re-intervention within a decade of use. Previous methods of seeding vascular grafts with autologous patient cells have been successful, however grafts constructed through these methods require copious amounts of preparation time prior to implantation. The growth or deposition of endothelial cells onto the luminal surface of the prosthetic vessel may be critical for optimal physiological integration of a vascular scaffold to the host environment. An alternative approach in the field of vascular tissue engineering includes the in situ endothelialization of acellular vascular scaffolds by capturing circulating endothelial progenitor cells (EPCs) and endothelial cells (ECs) directly from the blood stream through biofunctionalized vascular grafts.
METHODS – Two-dimensional vascular scaffolds, designed to be analogous to the vascular lumen, were electrospun with a 1:1 PCL/collagen solution. Experimental scaffolds were engineered through surface modification to immobilize EPC-specific antibodies, which were compared to unmodified scaffolds serving as controls. The antibodies tested were EPC/EC surface markers, including CD31, vascular endothelial cadherin (VE-Cadherin), vascular endothelial growth factor receptor 2 (VEGFR2), and Von Willebrand factor (vWF). Microfluidic cell capture experiments utilizing mouse pancreas/islet endothelial cells (MS1s) were performed in triplicate for each antibody using previously optimized conditions. After cell capture efficacy of each antibody was assessed, antibodies were paired to examine synergistic cell capturing capabilities in the microfluidic environment. Cell capture results were analyzed through paired t-tests and ANOVA.
RESULTS – Preliminary data demonstrates that vascular scaffolds bioconjugated with EPC-specific antibodies capture circulating cells at a higher rate than unmodified electrospun scaffolds in a microfluidic environment. Compared to unmodified scaffolds, CD31- and vWF-bound scaffolds captured more circulating endothelial cells than the unmodified controls (22.00 ± 20.02 vs. 19.41 ± 30.95 and 37.25 ± 23.53 vs. 19.41 ± 30.95, respectively). Furthermore, VECadherin- and VEGFR2-bound scaffolds captured more cells than the unmodified scaffolds at a statistically significant level (47.33 ± 25.50 and 56.33 ± 40.33). The experimental trials to examine synergistic cell capturing capabilities and cell proliferation are currently being conducted.
CONCLUSIONS – This project has the potential to vastly improve upon existing models for tissue-engineered vascular grafting approaches. While the use of immobilized antibodies has previously been proven to be advantageous in capturing endothelial cells, comparisons between the efficacy of distinct antibodies has yet to be explored in an experimental setting. Furthermore, the use of multiple antibodies for a synergistic effect in honing endothelial cell attachment to the lumen of engineered vessels is a novel approach in the application of vascular engineering. This project contributes necessary knowledge to the field of tissue engineering in regard to the comparison of existing modalities. Additionally, successful demonstration of synergistic cell capturing capability is instrumental in the development of a multifunctionalized acellular vascular graft. 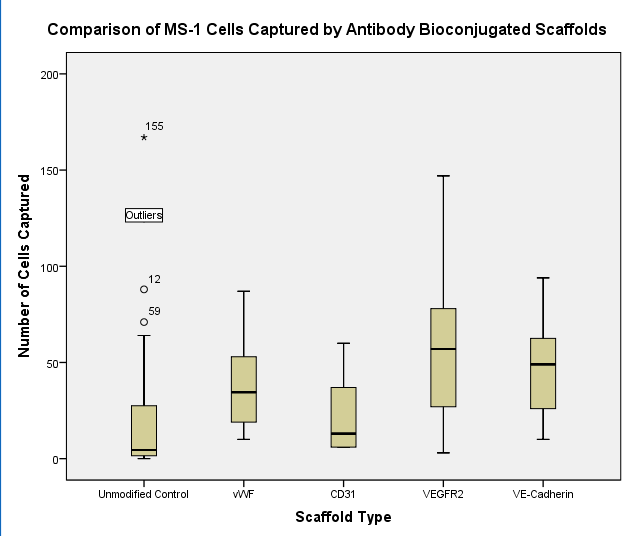
Back to 2019 Abstracts
