Early Results and Feasibility of Total Endovascular Aortic Arch Repair Using Three-Vessel Company-manufactured and Physician-modified Stent-grafts
Kyongjune B Lee, Jesus Porras Colon, Carla K Scott, Felipe L Pavarino, Mirza S Baig, Melissa L Kirkwood, Carlos H Timaran
UT Southwestern Medical Center, Dallas, TX
Objective: Total endovascular repair of aortic arch aneurysms is feasible in select patients. Unfortunately, and because of anatomic constraints, some patients are ineligible for company-manufactured devices. This study aims to evaluate the feasibility and early outcomes of total endovascular arch repair using three-vessel company-manufactured and physician-modified stent-grafts.
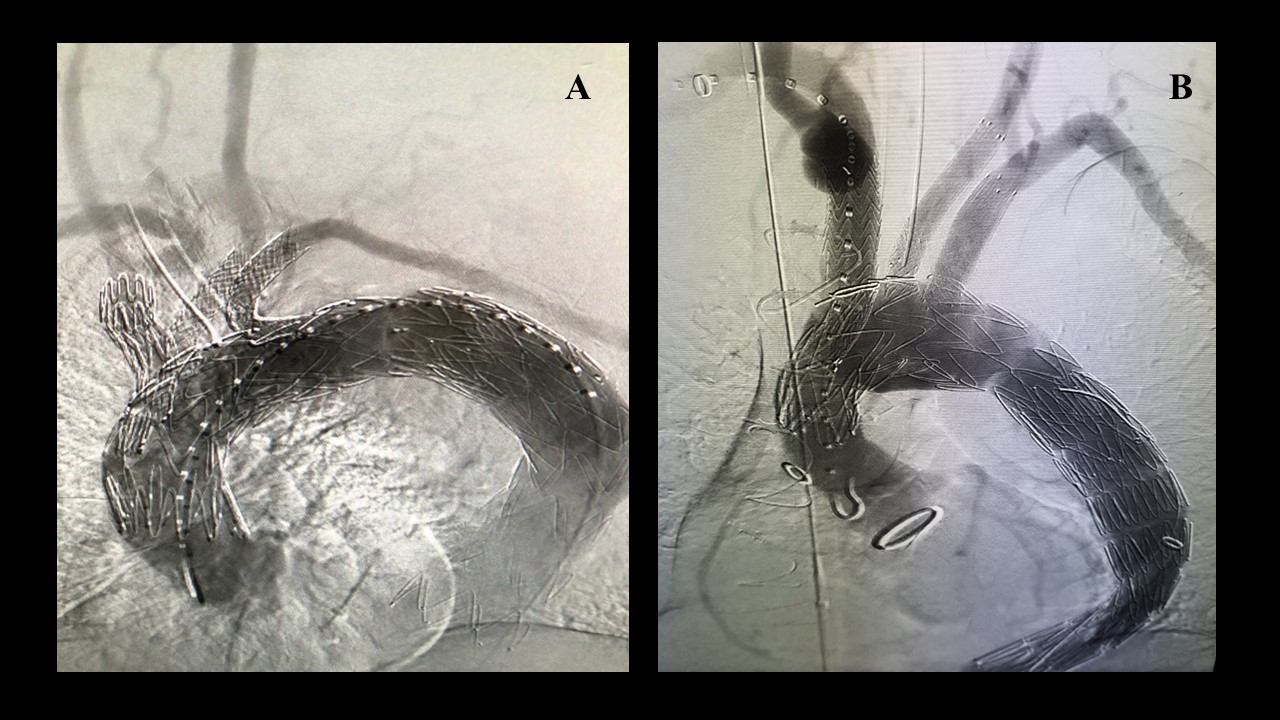
Methods: Patients unfit for open repair undergoing three-vessel total arch repair at a single institution from 2018 to 2021 were reviewed. Patients received either company-manufactured three-vessel inner branch devices (William Cook Europe, Bjaeverskov, Denmark) or physician-modified stent-grafts with inner branches or fenestrations (Fig 1). Three-vessel designs were used to incorporate the innominate, left common carotid, and left subclavian arteries. The antegrade inner branches in both devices were accessed via right brachial or carotid approach. The left carotid was accessed via carotid cutdown or femoral approach. The left subclavian artery was accessed via transfemoral approach. The study endpoints included technical success, patient survival, neurologic events, cardiac complications, reinterventions and target artery patency.
Results: Nine patients (seven male, 77%; mean age, 70 ± 7 years) underwent treatment of four degenerative (44%) and five chronic (55%) post-dissection aneurysms. Five patients were treated with company-made devices (Fig 1A) and four with physician modified stent-grafts (Fig 1B ). Patients were not eligible for company-manufactured devices because of severe aortic angulation (two patients), short proximal length from the sinotubular junction (one patient) or small access (one patient). Technical success was 100%. No in-hospital or postoperative deaths occurred during the median follow-up period of 260 days. There were no strokes, transient ischemic attacks, myocardial infarction, or spinal ischemia in the perioperative period. Major adverse events occurred in three patients (33%). Two (22%) vascular access complications and one (11%) acute kidney injury occurred. One (11%) patient required early reintervention for access complication. Mean length of stay was 7 ± 4 days. Computed tomography imaging obtained during the immediate postoperative period revealed endoleak in 6 (66%) patients, out of which five resolved spontaneously, and one required reintervention via left subclavian artery stenting. Target artery patency was 100% at the end of follow-up.
Conclusions: Three-vessel total endovascular aortic arch repair using company-manufactured or physician-modified stent-grafts is feasible with optimal early outcomes. Physician-modified stent-grafts are a feasible option for patients who do not meet anatomic criteria for company-manufactured devices.
Figure 1: (A) Total endovascular three-vessel aortic arch repair using custom made endograft (B) Total endovascular three-vessel aortic arch repair using physician modified endograft.
Back to 2022 Abstracts
