Important Differences in Real-world usage and Outcomes after TEVAR for Type B Aortic Dissection Indicate a need for Registry Involvement in Post-market Assessment of Vascular Devices
Charles Adam Banks1, Juliet Blakeslee-Carter1, Zdenek Novak1, Grace Wang2, Jack L Cronenwett3, Mark F Fillinger4, Joseph V Lombardi5, Rodney A White6, Ali Azizzadeh7, Adam W Beck1
1University of Alabama at Birmingham, Birmingham, AL;2University of Pennsylvania Hospital, Philadelphia, PA;3Division of Vascular Surgery, Dartmouth- Hitchcock Medical Center, Lebanon, NH;4Dartmouth College Geisel School of Medicine, Lebanon, NH;5Vascular and Endovascular Surgery, Professor of Surgery of Cooper Medical School of Rowan University, Birmingham, AL;6UCLA School of Medicine, Torrance, CA;7Cedars Sinai Smidt Heart Institute, Los Angeles, CA
Introduction and Objectives:The FDA often requires post-market approval studies (PMA) to assess device performance after market approval. However, PMAs often employ strict enrollment criteria that may lead to significant differences in PMA patients compared to real-world practice. Registries such as the Vascular Quality Initiative (VQI) provide an innovative method for evaluation of devices after approval, and are more indicative of real-world practice and outcomes.
The VQI TEVAR for Type B aortic dissection (TBAD) Post-Approval Study (PAS) began after market approval of two thoracic devices in 2013, evaluating consecutive TBAD patients treated at 49 centers nationwide. Enhanced data collection provided the ability to compare PAS patients to those enrolled in the device pivotal studies, which included only complicated TBAD patients. This study compares demographics, aortic anatomy, operative outcomes, and mortality from pivotal trial data and PAS complicated TBAD patients.
Methods:The VQI PAS cohort was filtered for patients undergoing TEVAR for acute complicated TBAD (N=146). Pivotal trial data was provided by both companies and aggregated for comparison (N=100, 50 patients per company). Patient demographics, presentation, anatomic elements, aortic reintervention rates and 30-day, 1-year, and 5-year survival were compared. Patients were also stratified separately by malperfusion or rupture presentation.
Results: Demographic and comorbidities were similar among the cohorts. Proximal neck length and diameter, as well as distal neck length differed significantly between the cohorts (Table). Distal extent of dissection affected the iliac arteries in a higher proportion of pivotal trial patients (62.6% vs 50.7%; p=<0.004). Proximal extent of dissection affected zones 0-3 in higher proportion of VQI patients (95.2% vs 51.5%; p<0.001).
Intraoperative adverse events were similar between the cohorts. Intraoperative endoleaks were more prominent within pivotal trial patients (4% vs 0; p=0.026). Postoperative adverse event rates were similar between the cohorts with increased proportion of postoperative lower extremity paresis and spinal cord ischemia within the pivotal trial cohort (Table).
Mortality at 30-days was 14.4% for VQI and 8% for the pivotal studies (p=0.16), with significant difference in 1-year mortality (VQI-24.7% vs Pivotal-13% (p=0.034). No significant difference was observed in 5-year mortality (VQI-28.1% vs. pivotal-28%; p=0.912) (Table & Figure). When comparing rupture patients only, 30-day survival (Pivotal-90.5% vs VQI-66.7%; p=0.049) and at 1-year (Pivotal-76.2% vs VQI-52.8; p=0.05) were different, although there was no difference at 5-years (Pivotal-61.9% vs VQI-50%; p=0.347).
Conclusions:This study demonstrates that patients treated in real-world practice were different from pivotal trial patients in not only presentation, but also anatomy and outcomes when compared to pivotal studies. These data demonstrate the importance of true real-world assessment of device usage and performance after market approval, and highlight the utility of registry involvement in post-
market assessment as well as the importance of collaboration between FDA, industry and clinicians. 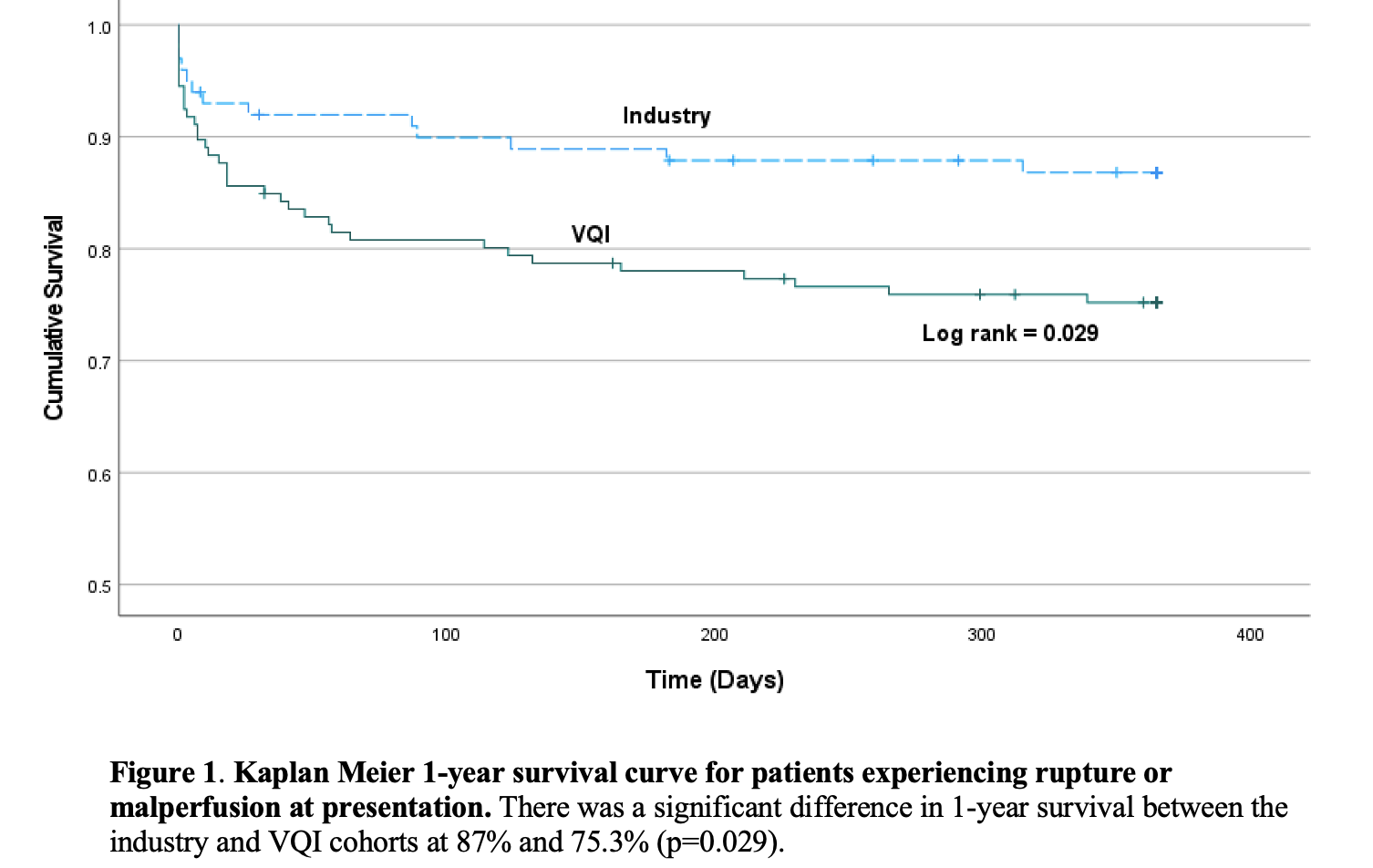
| Variable | VQI (n=146) | Pivotal Trials (n=100) | p-value |
| Age (yrs) | 58.0 ± 13.0 | 57.4 ± 12.2 | 0.193 |
| Male Gender | 111 (77%) | 77 (77%) | 0.88 |
| White | 97 (66.4%) | 59 (59%) | 0.234 |
| Time from Diagnosis to Treatment | 4.1 ± 5.4 | 3.1 ± 4.1 | 0.112 |
| Ruptured Presentation | 36 (24.7%) | 21 (21%) | 0.541 |
| Max Diameter (mm) | 41.4 ± 11.7 | 42 ± 9.1 | 0.49 |
| Proximal Neck Length (mm)* | 21.2 ± 10.1 | 22.6 ± 29.8 | <0.001* |
| Proximal Diameter (mm)* | 31.9 ± 4.6 | 30.8 ± 3.6 | 0.026 |
| Distal Seal Length (mm)* | 40.3 ± 37.6 | 19.2 ± 17.5 | 0.001* |
Back to 2023 Display Posters


